Sebia's Serum Free Light Chain Assay
For the diagnosis and monitoring of Monoclonal Gammopathies and AL Amyloidosis
Defining a New Standard of Excellence
in Serum Free Light Chain Testing
Superior Clinical Results
17% higher specificity compared to immunoassay, while reducing the impacts of antigen excess and polymerization
Decreased Repeat Rate
Up to 4x fewer repeats compared to immunoassay, saves valuable laboratory resources
Request More Information
If you would like to receive more information about Sebia's Serum Free Light Chain assay, our Team is here to support you.
Please fill out the below form to be contacted by a Sebia expert and learn more.
Multiple Myeloma
Multiple Myeloma is a plasma cell malignancy in which monoclonal plasma cells proliferate in bone marrow, resulting in an overabundance of monoclonal paraprotein (M protein), destruction of bone, and displacement of other hematopoietic cell lines.
Diagnosis and Management
New Patients
In 2023, an estimated 35,730 adults (19,860 men and 15,870 women) in the U.S. will be diagnosed with Multiple Myeloma. This has increased from 2022 where the number of reported diagnoses were 34,470.
Time to Diagnosis
22% of patients diagnosed with Multiple Myeloma need 6 or more appointments before being referred to a specialized physician to test for the disease.
Diagnosis and Monitoring
More recent evidence suggests that patients who have a high-risk type of Smoldering Multiple Myeloma (SMM) should be offered early treatment and monitoring to properly diagnose and support an individual through the progression of the disease.
What are Free Light Chains?
Free light chains (FLCs) are immunoglobulin light chains that are circulating in serum in a free and unbound state. As a clinical biomarker, serum Free Light Chain is used as a key indicator in the monitoring, and diagnosis of Monoclonal Gammopathies.
The Value of sFLC Testing
-
The Level of serum FLC is used as an aid in the diagnosis, prognosis, and monitoring of Multiple Myeloma patients and related disorders.
-
Ratio FLC value (kappa FLC/ Lambda FLC) compared to reference ranges may indicate the presence of plasma cell disorders like Multiple Myeloma or AL Amyloidosis.

Industry Guidelines for Compliant Testing
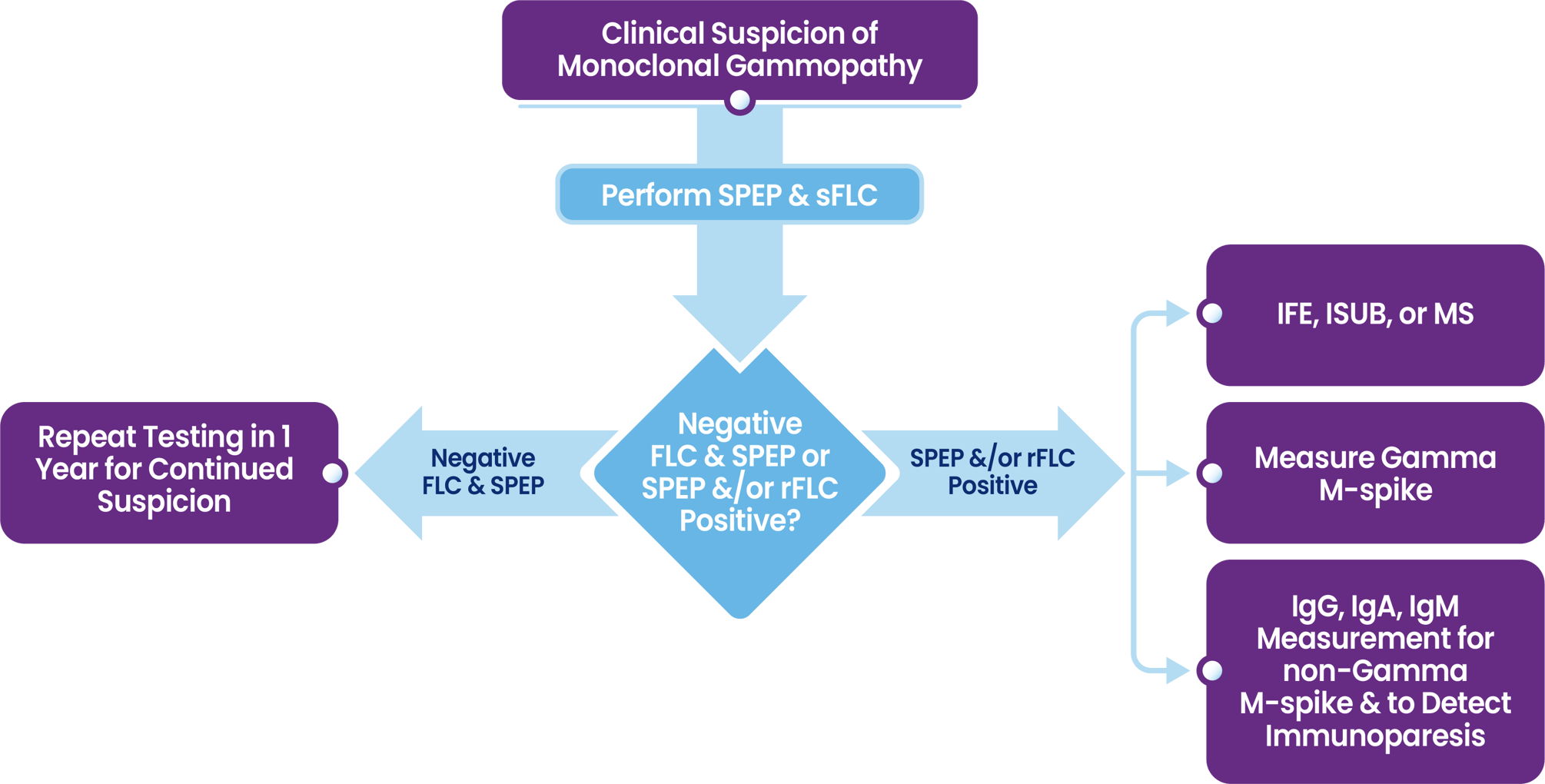
-
For the initial detection of monoclonal immunoglobulin protein (M-protein) in all patients with suspected monoclonal gammopathies (MG)
-
For the initial detection of M-protein in all patients with suspected Amyloid Light chain (AL) Amyloidosis
Sebia's sFLC Solution
Sebia is the only full solution provider that can support your Lab in the diagnosis and monitoring of monoclonal gammopathies. Offering the most accurate results utilizing leading-edge technology, at a competitive price, to help your Lab achieve all clinical and business objectives.
sFLC Assay on Automated ELISA
ELISA Simplified
-
Automated ELISA Method
Eliminates manual pipetting, dispensing, washing, and incubating, offering convenience, higher throughput, decreased human error, and greater reproducibility while utilizing a well-established and trusted method.
-
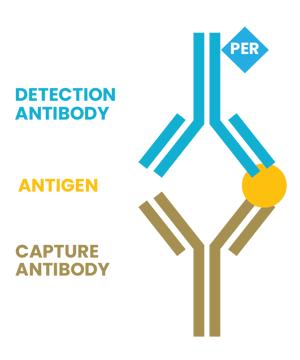
ELISA Sandwich
ELISA can measure elevated levels of free light chains in plasma cell disorders using two monoclonal antibodies specific to kappa and lambda light chain types, which bind to different parts of the target protein or antibody, creating a "sandwich" structure that can be detected with a signal-generating enzyme.
Clinical Advantages
Superior Analytical & Clinical Performance
-
Sebia FLC assays shows strong sensitivity and specificity
Sebia's FLC assays have demonstrated comparable clinical sensitivity with the predicate for the diagnosis of MM (96.6% on 177 samples from patients diagnosed with MM), better clinical sensitivity for the diagnosis of AL (91.0% on 144 samples from patients diagnosed with AL) and better clinical specificity (85.1% on 189 non-myeloma/non-amyloidosis subjects with various clinical conditions)*.
*Source: data from FDA 510k K210623, November 2022
Proven Clinical Equivalence and Commutability
-
>20 and >100 Biomarkers
A study performed at the Mayo Clinic demonstrated that Sebia’s FLC assay is clinically equivalent and commutable to The Binding Site’s™ Freelite® assay at the >20 and >100 sFLC biomarkers, which are vital cut off points to a clinician in the diagnosis and monitoring of a Multiple Myeloma Patient.
-
Harmonization Between Assays
Despite the analytical differences between Sebia's sFLC assay and Freelite® assay, the existing FLC criteria for Multiple Myeloma and SMM is applicable across both assays.
Reduced Impact from Antigen Excess and Polymerization
-
Reduced Impact from Antigen Excess
Up to 4x times fewer repeats compared to current methods, resulting
in potential significant cost savings -
Reduced Impact from Polymerization
Sebia sFLC Assay showed a 1.4% greater sensitivity and a 17% greater specificity compared to other sFLC assays on the market
-
Jacobs JFM et al., 2017: "both Freelite and N Latex nephelometric assays substantially overestimate the concentration of monoclonal FLC in samples with high titers, which is caused by FLC polymerisation. In this study we confirm these data, and demonstrate that the Freelite monoclonal FLC concentration was consistently, and on average 12-fold, overestimated compared to quantification by electrophoresis"
Discover More
If you're interested in learning more about Sebia's Serum Free Light Chain Assay, please explore the variety of clinical resources Sebia has developed.

On-Demand Webinar
Recent Insights into Serum Free Light Chain Assays for Plasma Cell Disorders

Clinical Evidence
Sebia's FLC Assay shows better clinical specificity than the FreeLite assay

Clinical Evidence
Sebia's assay demonstrates clinical equivalence in a study performed at The Mayo Clinic

